Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is the most common pediatric cancer. The outcomes of young adults with B-ALL are poor, with an event-free survival (EFS) rate of <50%. The prognosis of children with certain types of B-ALL is also poor with an EFS rate of 70%. Therefore, there is a need for new treatments for these children with high-risk B-ALL. RNA splicing alternations have been suggested to contribute to the initiation and/or maintenance of cancer. In B-ALL, dysregulated splicing is reported to be associated with drug resistance and disease relapse. SF3B1 is a core component of the spliceosome and an essential protein in the RNA splicing process. SF3B1 inhibition is therapeutic in many cancers, including myeloid malignancies and T-cell ALL. However, there have been no reports studying the efficacy and the function of spliceosome inhibitors including SF3B1 inhibitors in B-ALL. In this study, we determined the therapeutic potential of pladienolide B (Plad-B), an SF3B1 inhibitor, in B-ALL.
Methods
SF3B1 mRNA and protein expressions were determined in the cell lines Reh and JM1 and 14 patient samples (standard-risk: 7 samples, high-risk: 7 samples), by qRT-PCR and Western blotting, respectively. Efficacy of Plad-B was tested in two B-ALL cell lines, CD34 positive hematopoietic stem cells (HSCs), and three high-risk patient-derived xenograft (PDX) samples (sample#1: age < 1 year old, sample#2: age > 10 years old, sample#3: relapse), by an MTS assay. In vivo efficacy of Plad-B was tested using an Reh xenograft model and a PDX model. Function of Plad-B was assessed in cell lines by a cell cycle assay, Annexin V assay with flow cytometry, and Western blotting of cleaved PARP and cleaved caspase-3. To determine the changes in the splicing events and apoptosis-associated genes, we are performing RNA-seq at 15min, 30min, 60min, and 24 hours after the treatment.
Results
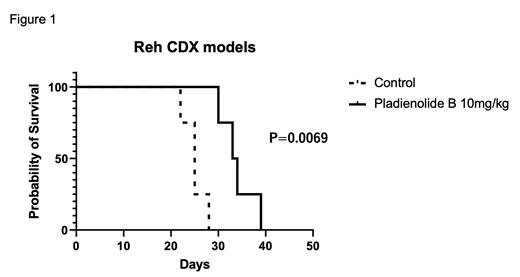
SF3B1 mRNA and protein expressions were significantly higher in Reh and JM1 than in hematopoietic monocular cells and CD34-positive HSCs (p < 0.05). SF3B1 protein, but not mRNA, expressions were higher in primary patient samples than in hematopoietic monocular cells and CD34-positive HSCs. There was no significant difference in the mRNA and protein expressions between standard-risk and high-risk groups. Plad-B treatments showed significant cytotoxicity in both Reh and JM1 cell lines with IC50 of 1.2nM and 0.6nM, respectively, at 48 hours after treatment. Plad-B did not show cytotoxicity at the same tested concentrations in CD34-positive HSCs. Plad-B treatments showed similarly significant cytotoxicity in three harvested PDXs with IC50 of 0.7nM, 2.5nM, and 1.1nM, respectively, at 48 hours after the treatment. Plad-B, at a dose of 10 mg/kg 2 times on 1 and 2 days, significantly prolonged survival in the Reh xenograft mouse model, with a median survival time of 33.5 days compared with 25.0 days in the control group (p < 0.05) (Figure 1). Plad-B, at a dose of 10 mg/kg 4 times on 1, 3, 5, and 7 days, prolonged survival in the PDX mouse model (sample#1), with a median survival time of 38.0 days compared with 32.0 days in the control group (p = 0.14). Cell cycle arrest was observed at 24 hours after Plad-B treatment in both Reh (Control: 12.6%, Plad-B 3nM: 23.8% in G2/M phase, p < 0.001) and JM1 (Control: 20.6%, Plad-B 3nM: 35.1% in G2/M phase, p < 0.01). Plad-B caused significant cell apoptosis in Reh (Control: 3.2%, Plad-B 3nM: 24.1% with Annexin V positive cells, p < 0.001) and JM1 (Control: 5.9%, Plad-B 3nM: 31.2% with Annexin V positive cells, p < 0.001) cells at 24 hours after treatment. Plad-B also caused significant cell apoptosis in harvested PDX sample #1 (Control: 29.8.%, Plad-B 3nM: 47.6% with Annexin V positive cells, p < 0.001) at 24 hours after treatment. Western blotting of anti-cleaved PARP and anti-cleaved caspase-3 showed that the expression of cleaved caspase-3 and cleaved PARP were significantly augmented in Reh and JM1 cells after Plad-B treatment. RNA seq data is currently being analyzed.
Conclusions
SF3B1 is highly expressed in B-ALL cell lines and in primary patient samples regardless of the risk group. Plad-B showed significant cytotoxicity in B-ALL leading leukemia cells to G2/M cycle arrest and apoptosis. Plad-B treatment demonstrated in vivo therapeutic efficacy in pre-clinical B-ALL animal models. These data suggest therapeutic potential of SF3B1 inhibition in B-ALL including high-risk patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal